Abstract
Background: Intravenous immune globulin (IVIG) is a plasma-derived blood product that is used as a treatment for various autoimmune diseases. The anti-CD20 monoclonal antibody rituximab has similar immunomodulatory properties and mechanisms of action. We did a systematic review and meta-analysis to determine the efficacy and safety of rituximab across a broad range of autoimmune diseases with a view to investigate this agent as an alternative to IVIG.
Study design: Systematic review and meta-analysis.
Methods: We identified the most common indications for IVIG (besides immune replacement therapy) from a recent Ontario audit based on total amount of IVIG used. Starting with the highest users, these were: chronic inflammatory demyelinating polyneuropathy (CIDP); immune thrombocytopenia (ITP); myasthenia gravis (MG); multifocal motor neuropathy (MMN); Guillain-Barre syndrome (GBS); systemic lupus erythematosus (SLE); Sjogren's syndrome (SS); and pemphigus vulgaris (PV). Next, we did a systematic review of rituximab for each of these conditions by searching MEDLINE, EMBASE and the Cochrane Library until July 2016. Randomized controlled trials (RCT) and observational studies of ≥10 adult patients were included. The primary outcome was clinical response at 6 months from RCTs across all conditions. Secondary outcomes were clinical response for each condition and adverse events. Pooled relative risk (RCTs) or pooled proportions (observational studies) were calculated using fixed and random effects models. Risk of bias was assessed for each study using the Cochrane Collaboration tool.
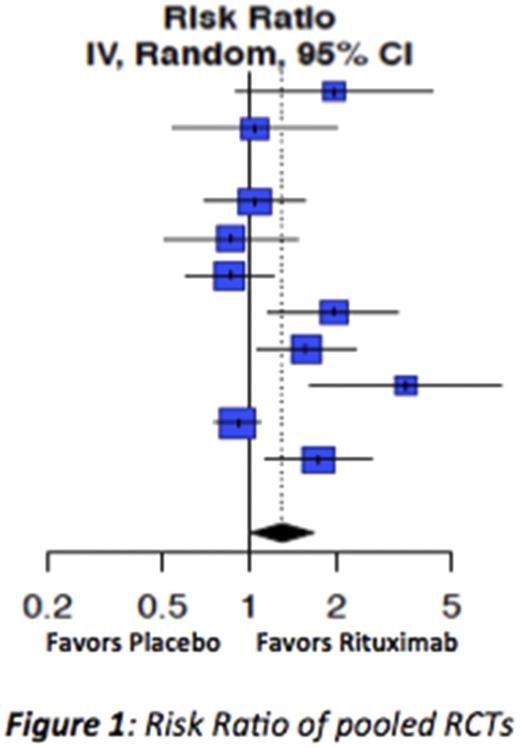
Results: We identified 108 studies for the target conditions (n=3536 patients) including 10 RCTs (n=1044). Most studies were in ITP (n=1543), SLE (n=1192), PV (n=504) and SS (n=200). There were 4 studies in MG (n=66), 2 studies in CIDP (n=31) and none in GBS or MMN. Risk of bias in 9 of 10 RCTs was low. Response to rituximab was 30% higher than controls (RR=1.30, 95% CI 1.01, 1.67) based on pooled results of 10 RCTs in ITP, SLE, PV and SS. Pooled proportions for disease-specific responses ranged from 48% (95%CI 30% -66%) for CIDP to 94% (95% CI 88% -98%) for PV. Adverse events were mild.
Conclusion: Rituximab is an effective immune-modulating treatment and may represent an alternative to IVIG for some conditions. Few studies have been done in the rare autoimmune neurological disorders despite these being among the most frequent indications for current IVIG use.
Arnold: Bristol Myers Squibb: Research Funding; Novartis: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; UCB: Consultancy; Dova: Consultancy; Rigel: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal